|
From June 2023 to September 2023, I worked in Professor Dietmar Manstein's lab in the Institute for Biophysical Chemistry at Hannover Medical School in Hannover, Germany.
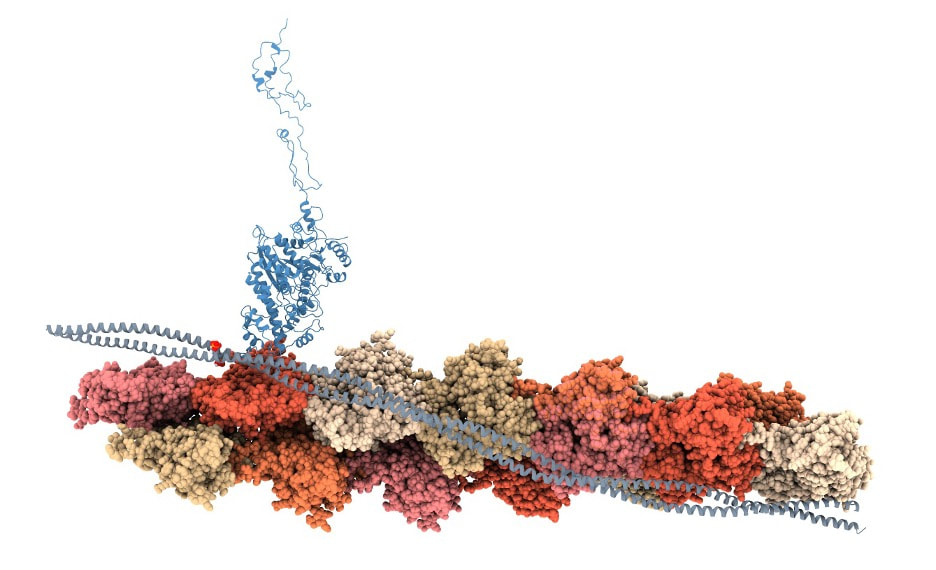
The cytoskeleton is a critical part of giving cells structure, and disfunction in cytoskeletal proteins can lead to malignant tumors and faulty immune cell function, among many other effects. Therefore, it is important to understand how cytoskeletal proteins are regulated. Actin is a critical cytoskeletal protein, the filaments are stabilized by tropomoysin, a coiled coil protein found ubiquitously in animal cells. Since it is hypothesized that post-translational modifications of the many different isoforms of tropomyosin may drive different functional properties of actin filaments, my project aimed to investigate the effect of phosphorylation on several different tropomyosin isoforms. I first picked a few different isoforms and phosphoylation sites of interest based on the literature and then produced phosphomimetic proteins where the wildtype residue was mutated to a glutamic acid, a negatively charged amino acid, in order to mimic phosphoylation. After purifying these constructs, I tested the mutations' effect on actin binding and myosin (a cytoskeletal motor protein) using various biochemical assays, microscopy assays, and in silico methods like molecular dynamics simulations. You can see the model we produced of the whole system to the right. |
|